sk life science xcopri
NDA subsequent development and commercialization. The most common side effects of XCOPRI include feeling sleepy and tired dizziness double vision and headache.
Sk Biopharmaceuticals Launches Epilepsy Drug In Us Pharma 기사본문 Kbr
9 2022 PRNewswire -- SK Biopharmaceuticals Co Ltd.

. The story starts about 2005 when cenobamate otherwise known as YKP3089 was developed by South Korean company SK Biopharmaceuticals hoping to develop it as an anti-anxiety drug. Laboratoires Paladin inc une filiale dEndo International plc a annoncé aujourdhui que Santé Canada a accepté sa demande de présentation de nouveau médicament PNM pour les comprimés. SK Life Science Inc.
2020년 5월 미국에 출시한 세노바메이트미국 제품명. Before starting XCOPRI cenobamate tablets CV its important to discuss the potential benefits and risks with your doctor. There is a chance you may experience side effects.
SK바이오팜대표이사 사장 조정우이 연결재무제표 기준으로 2분기 매출 534억 원 영업손실 401억 원을 기록했다. Â 2022 SK Life Science Inc a subsidiary of SK Biopharmaceuticals Co Ltd. Subsidiary SK life science are global pharmaceutical.
Learn about XCOPRI dosing titration and administration to help get your epilepsy patients started on the partial-onset seizure medication. In March 2022 SK Life Science and Celerion agreed an out-of-court settlement with her family for an undisclosed sum. Continued success of XCOPRI development.
SK바이오팜대표이사 사장 조정우이 연결재무제표 기준으로 2분기 매출 534억 원 영업손실 401억 원을 기록했다. SK Life Science Inc. Announced that Health Canada has accepted Paladin Labs Incs filing of a New Drug Submission NDS for cenobamate as an adjunctive therapy for the.
세노바메이트 xcopri 미국 제품 사이트로 이동. SK Biopharmaceuticals is eligible to receive milestones for future approval and commercialization in Canada and Israel. SK바이오팜의 2022년 2분기 매출은 전년 동기 대비 123 증가한 것으로 뇌전증 치료제 세노바메이트의 매출 상승 및.
Xcopri는 sk바이오팜이 신약 후보물질 발굴부터 미국 식품의약국fda 허가까지 전 과정을 직접 진행하여 독자적으로 개발한 치료제 입니다. And was launched at the American Epilepsy Society AES annual meeting to bring prescribers into the lives of patients and start a discussion on teh company. Thats what the company SK Life Sciences one-of-a-kind completely immersive virtual experience has done through its brand Xcopri.
WHAT YOU NEED TO KNOW. Â 2022 SK Life Science Inc a subsidiary of SK Biopharmaceuticals Co Ltd. SK바이오팜의 2022년 2분기 매출은 전년 동기 대비 123 증가한 것으로 뇌전증 치료제 세노바메이트의 매출 상승 및.
XCOPRI is a federally controlled substance CV because it can be abused or lead to dependence. PANGYO South Korea Aug. The campaign is called Step into their shoes.
Global Life Science Investment Fund. SK Biopharmaceuticals and its US. Show Are You HCP.

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Sk Life Science Presents Long Term Data Of Xcopri Cenobamate Tablets Cv In Adults With Partial Onset Seizures At The American Epilepsy Society 2021 Annual Meeting

Sk 200 Pill Orange Round Pill Identifier Drugs Com

Xcopri Now Available For Partial Onset Seizures In Adults Mpr

New Drug Filing For Cenobamate Accepted For Review In Canada And Israel
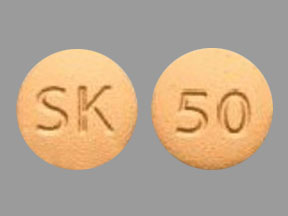
Sk 50 Pill Yellow Round Pill Identifier Drugs Com

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk
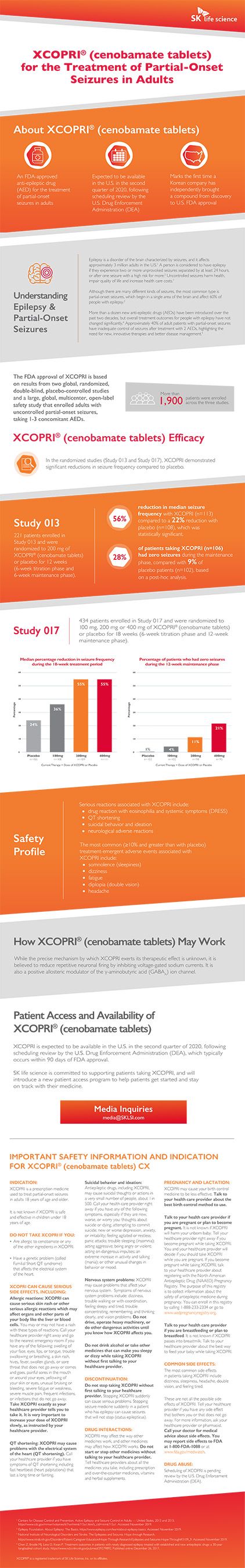
Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Sk Biopharmaceuticals Anti Epileptic Med Cenobamate Launched In Us Pulse By Maeil Business News Korea

Management Archives Sk Life Science Inc Sk Life Science Inc

Taking Xcopri What You Need To Know Xcopri Cenobamate Tablets Cv